New Stargardt Treatments- MD Stem Cells SCOTS2 or ALK-001 Vitamin A
Stargardt Retina
New treatments for Stargardt Macular Dystrophy compared
USA, November 10, 2022 /EINPresswire.com/ -- Stargardt Disease (SGTD) is a hereditary retinal disease caused by mutations of the ABCA4 gene, impairing processing of Vitamin A. Buildup of these byproducts is thought to be the cause of retinal cell damage and blindness.
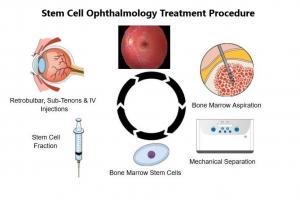
The Stem Cell Ophthalmology Treatment Study II or SCOTS2 has achieved notable success in treating many eye diseases including Stargardt. Developed by MD Stem Cells, the procedure uses the patient’s own Bone Marrow Stem Cells (BMSC) to treat retinal and optic nerve diseases. BMSC have been shown to protect other cells and help block cell death in those that are stressed. BMSC have also been shown to turn into certain retinal cells.
WHAT’S NEW:
MD Stem Cells treated Stargardts patients in SCOTS. A high percentage had improved binocular vision or their vision stayed stable in follow up. Access their published paper on Stargardt Disease to understand these very positive results.
A separate company modified Vitamin A into a drug called ALK-001. They treated a group of patients with ALK-001 once a day for 2 years to see if this would help avoid spots in the retina called atrophy.
ACTUAL RESULTS:
In SCOTS, the end point (what was being measured) was the patient’s vision or visual acuity. 94.1% of patients showed improved or stable vision with both eyes in the one year follow up period. A total of 85.3% of treated eyes showed benefit with 61.8% showing visual improvement and 23.5% remaining stable in the follow up. The results were highly statistically significant- the gold standard in medicine that shows the procedure was responsible for the benefits. There were no adverse events or complications with the treatment.
In the ALK-001 study, researchers used the change in atrophy and not vision as an endpoint. ALK-001 did not completely stop the atrophy, but did slow it by 21% over the 2 year study , or .35 mm2 in a year (size similar to small zero in % sign). Vision did not change.
HOW DO THE TREATMENTS COMPARE:
The Stem Cell Ophthalmology Treatment Study II ( SCOTS2) uses the patient's own stem cells for treatment. A small portion of the patient’s bone marrow is taken, they separate the active stem cell population, and treat the patient with 2 ocular injections followed by intravenous- all under a short period of anesthesia- so no pain. There is strong and statistically significant evidence that the treatment can improve visual acuity or halt loss. SCOTS2 is a procedure. All patients accepted receive treatment- there is no sham or placebo group.
ALK-001 is a new drug. The drug is not yet approved by FDA and only available in clinical study. In the report, there was a placebo ( pill without medication) used on some of the patients who enrolled. It is common for drug studies to have placebo groups where patients and doctors do not know who is receiving the active drug.
RESULTS IN OTHER EYE DISEASES:
SCOTS2 treats a number of different retina and optic nerve diseases. In addition to Stargardt, eye disease showing benefit included AMD (macular degeneration), Retinitis Pigmentosa, Usher, Cone-Rod, Rod-Cone, Cone dystrophy, Bests dystrophy, non-perfusion retinopathy, retinal injury, retinal inflammation such as POHS or choroiditis, certain diabetic retinopathies and certain post Retinal Detachment vision loss. A number of optic nerve conditions have also responded including Glaucoma, NAION, LHON, ADOA, optic nerve ischemia/ neuropathy/ optic neuritis/optic atrophy/ and optic nerve compression. To our knowledge, ALK-001 is only being studied for Stargardt Disease at this time.
TAKE HOME POINTS:
MD Stem Cells has treated many patients with a variety of eye diseases. It has published multiple medical and scientific papers. Patients and health care providers should feel reassured that the doctors associated with MD Stem Cells have the knowledge and experience to assess, and if deemed appropriate, carefully treat your retinal or optic nerve condition. All patients receive treatment- there is no placebo or sham treatment.
New drugs are highly important but may take years of careful clinical research to finally be approved. This process may require patients to take a drug for long periods- some may be given a placebo.
I WANT MORE INFORMATION:
Receive information about participating in SCOTS2 by emailing Dr. Levy at stevenlevy@mdstemcells.com with your name, cell phone, email address and brief history of their disease. You may also use the contact us page on www.mdstemcells.com or call directly 203-423-9494. MD Stem Cells has no grant support and is not a pharmaceutical company; this is a patient sponsored studies and the patients pay for both treatment and travel.
Steven Levy MD
MD Stem Cells
+1 203-423-9494
stevenlevy@mdstemcells.com
Visit us on social media:
Facebook
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.